
“It involves the South Korean firm's first application of quantum-dot tech - screens that feature crystals more than 50,000 times smaller than the width of a human hair that can generate vivid colours and are capable of being more than twice as bright as conventional TV screens (BBC)”.
In January 2015, an unprecedented announcement took place at CES - the world's largest tech show. After 10+ years of research, Samsung Electronics announced a line of high-performance UHD TVs that use its own quantum dot technology to boost color. The launch was lauded for the performance of quantum dot TVs and captured global media coverage. Five years later, Samsung Electronics introduced its next-generation QLED 8K line at CES 2020, immediately captivating those visiting the booth.
In October 2019, Samsung Display announced that it would invest $11 billion in the world’s first dedicated Quantum Dot display manufacturing facility and start mass-production in 2021. This begs the question: What exactly is the hype over Quantum Dot technology?
Same Material, Different Color?
A Quantum Dot is a nanoscale (one nanometer is 10 -9 meter or a millionth of a millimeter) particle of semiconducting material. To summarize succinctly, quantum physics refers to the laws of physics that apply to the physical properties of nature at the scale of very small particles.
Atoms, molecules, or smaller particles work in mysterious ways that are comparable to what humans normally see and think intuitively. Nanomaterials are a good example of this unusual characteristic: Even when produced with the same material and identical procedures, the color of the light emitted by nanoparticles vary according to their sizes.
Let’s imagine a bar of pure gold (Au) sitting in front of us. Regardless of how big or small the bar is, most people will agree that the color remains the same - it’s gold. However, if we were to split the gold bar into pieces to the point where they are made into nanometer(nm)-sized grains, we will witness color change. At 7nm, gold particles emit red, green at 5nm, and blue at 3nm. Likewise, a Quantum Dot (a nanoscale semiconductor particle) also shifts colors based on its size.
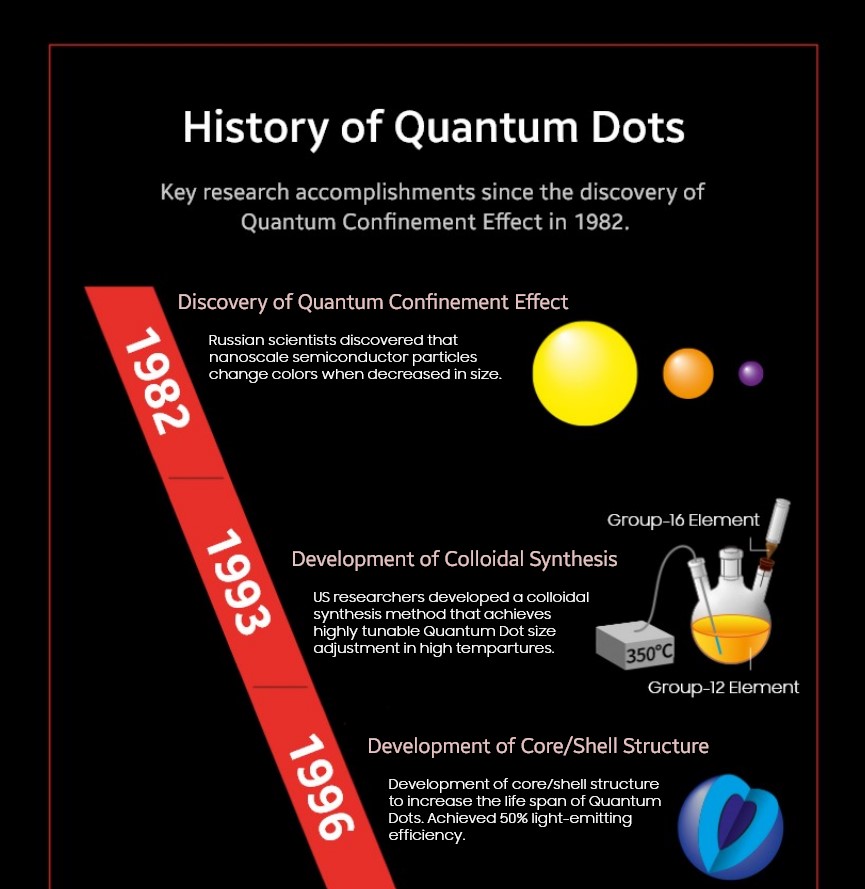
Can you imagine what it must have been like to be the first person to discover this astonishing phenomenon? In 1982, a number of Russian scientists identified this trait in Cadmium(Cd) - a core element in today’s semiconductor materials - found in stained glass windows decorating the walls of ancient cathedrals and began to investigate further.
What determines color in a Light Emitting Diode (LED) is the movement of electrons in semiconductors. The color of light is determined by the energy of the bandgap. And the energy fluctuation of this electron when external energy such as current or light is injected is uniquely determined for each substance.
For example, in LEDs, Gallium arsenide (GaAs) has a range of electronic energy fluctuations that produce red, Gallium Arsenide Phosphide (GaAsP) yellow, and Gallium Nitride (GaN) blue. However, if the particle size is reduced in nanometers, energy levels become discrete. The relatively large particles (7nm) have small variations, while the relatively small particles (2nm) have large variations.
Large energy fluctuations mean that bandgap size increases, and thus energy emitted increases, producing blue. Conversely, in relatively large particles in units of nm, the bandgap is decreased and emitting red. The quantum confinement effect - as the Russians coined it - occurs when the particle size is too small to be comparable to the wavelength of the electron.
Bae WanKi , professor of nanotechnology at Sungkyunkwan University, explained, "If the electrical and optical characteristics of existing semiconductors were determined only by the composition/structure of materials, the advent of Quantum Dot technology added another dimension - size," and added, “Now we will be able to utilize more variety of existing semiconductor materials.”

