
Core, Shell, and with Ligands
A quantum dot (QD)’s introduction to the semiconductor industry was deemed revolutionary for its key characteristic - emitting different colors based on its measurement. Unlike its predecessors, with one particular material, it now became possible to create various colors ranging from blue to red simply by adjustment of size.
Due to its many promising possibilities, Quantum Dot research was actively carried out in the 1990s. In 1993, "Colloid Synthesis" was developed, allowing people to precisely control the size of quantum dots for the first time.
Method of synthesizing is straightforward: Heat group-12 of the periodic table elements (zinc, cadmium, etc.) with group-16 elements (sulfur, selenium, etc.). These are commonly used semiconductor materials, but additional sources are needed to stabilize the particle surface. This is because the smaller particles are, the greater impact of the particle surface is.
For example, a cube with a side length of 10cm has a ratio between volume and area of 10:6, while a cube with a side length of 1cm has a ratio of 1:6. The smaller the size, the greater the proportion of its surface. “The large surface-to-volume ratio of QDs places a substantial importance on the composition and structure of the surface in defining the physical properties that govern the concentration, motion, and dynamics of excitations and charge carriers in QD (Science).”
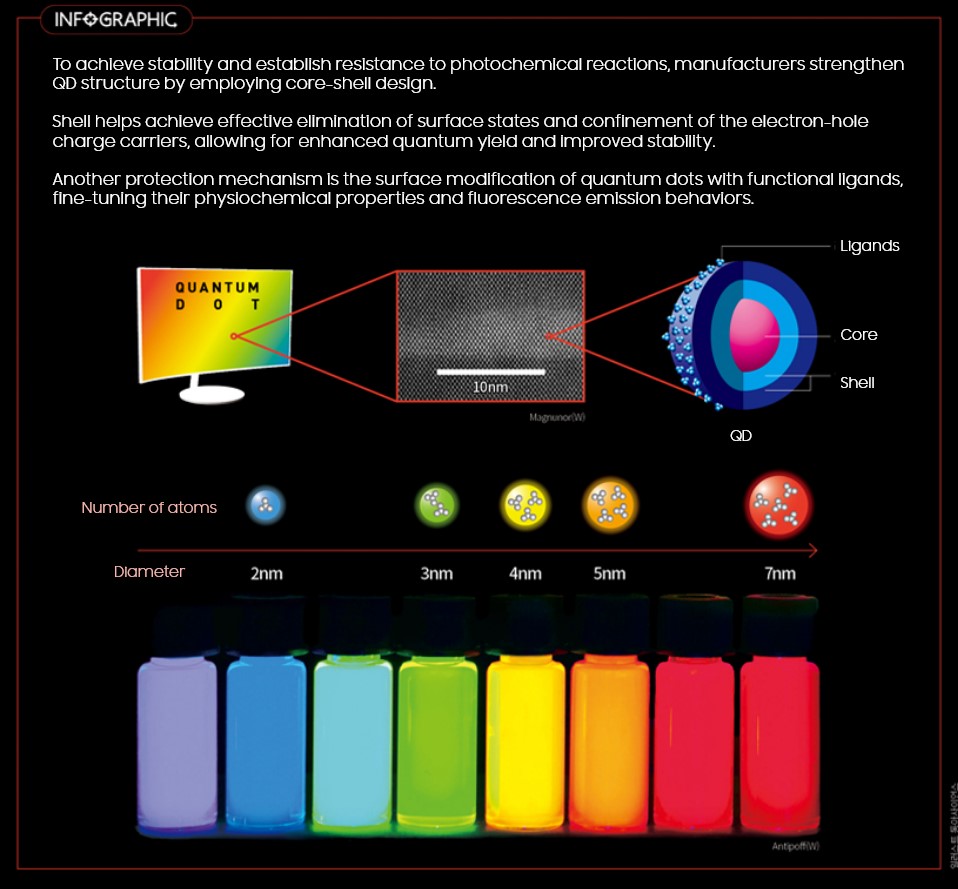
With this in mind, Quantum Dots are created to be harnessed at its maximum capacity of electric and optical by having the core wrapped in a shell with ligands* attached as the final layer.
Wan Ki Bae, professor of nanotechnology at Sungkyunkwan University, noted: “Understanding the types of ligands and methods of ligands attachment is essential in QD. Other factors like temperature and reaction time also play a determinant role in controlling lifespan and size of Quantum Dots.”
Recently, the harmful environmental impact of cadmium (group-12 element) has pushed development towards new approaches in utilizing group-13 elements (gallium, indium, etc.) with group-15 elements (phosphorus, arsenic, etc.) instead of group-12 elements and group-16 elements. Professor Bae said, "Elements in group-12 and 16 combine easily in ionic bonds, but group-13 and 15 elements need to be covalently bonded, so it is more difficult to make compounds. Currently, we are exploring new methods - from using highly reactive elements to increasing reaction temperature in a way that doesn't damage the particles"
Researchers and fellows from Samsung Advanced Institute of Technology at Samsung Electronics developed the Quantum Dot technology available for commercial purposes, utilizing group-13 elements and group-15 elements, and had the study published by Nature in November 2019**.
The research team synthesized the group-13 element Indium (In) and group-15 element Phosphorus (P), and then covered it with double shell made of Zinc selenide (ZnSe) and Zinc sulfide (ZnS). "Quantum-dot research conducted by Samsung Electronics and other Korean universities/institutions is noteworthy globally," according to Wan Ki Bae. "These accomplishments reflect on Korea’s continuous investment on nanotechnology for the past 20 years."
*Ligands: any atom or molecule bonded to a central atom, usually a metallic element, in a coordination or complex compound (Britannica)
**Won, YH., Cho, O., Kim, T. et al. Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature 575, 634–638 (2019). https://doi.org/10.1038/s41586-019-1771-5

