
Energy ‘Value’ Instead of Energy ‘Band’
There is a clear difference between Quantum Dot displays and other types of displays. Compared to other displays, QD displays can produce primary colors closest to the industry standard, and it can significantly reduce the mixing of different wavelength gamut. This can be credited to the fact that Quantum Dots, which are luminescent materials, are very small materials in which a small number of atoms are collected.
▶️ Read Previous Article: [Quantum Dot 101] #2 Lifelike Images ① How Quantum Dot Displays Produce Three Primary Colors!
Remember ‘energy levels’ from your high school chemistry class? First, think of a basic atomic structure. As shown in the illustration below, an atom has a central nucleus surrounded by electrons arranged in shells.
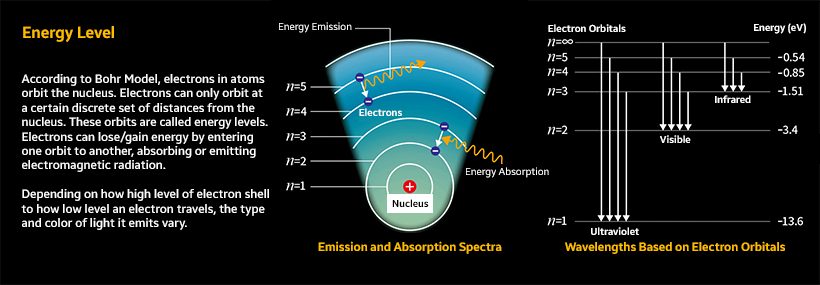
Electrons can move between shells. For instance, if an electron absorbs energy from external sources, it may become excited and move to a higher level of electron shell. However, if an electron loses energy, it may move to a lower level. The energy value of an atom varies depending on which number of electron shells the electron starts to move, and the energy level is called the total energy value that an atom can have.
Usually, when there is only one atom, the energy level is set to a certain number. However, when multiple atoms exist, the electron shells of each atom overlap, causing some electrons to slow down while others accelerate. This leads to a change in energy level.
What if the number of atoms increases? Energy values different from the existing energy level will appear, and eventually, the energy level will end up being expressed in a certain range rather than a specific value. This ‘range’ of energy values is referred to as an energy band. Most substances have energy bands because they are made up of many atoms.
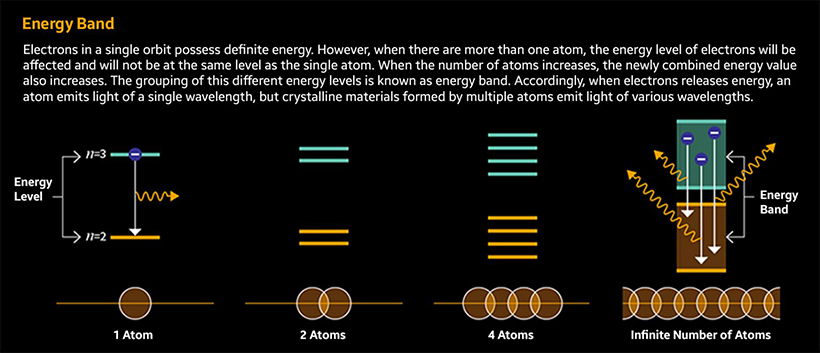
Now let’s apply everything we learned using Quantum Dot: Luminescent materials such as QDs are characterized with electrons that receive electricity or light energy externally, which then move to the outer electron shell and return inward after emitting light. This light emission is what we perceive with our eyes.
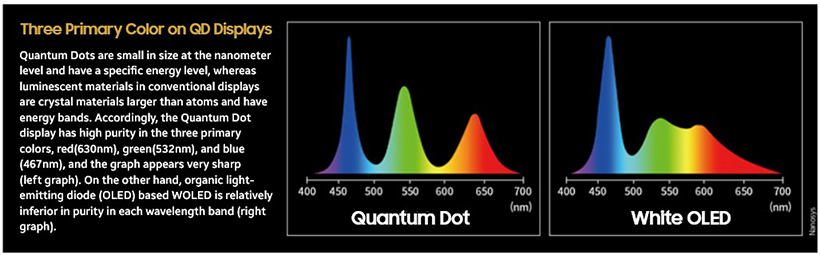
The reason why the color of the light emitted is different is that the amount of energy the electron loses when moving differs according to materials. Luminescent materials used in the display industry are composed of numerous atoms, and the energy emitted has a certain range, not an exact value. In the case of a luminescent material that emits 630nm of wavelength, it means that the main emission color is 630nm and the wavelength of the actual emitted light is in a wider range.
However, a Quantum Dot consisting of a few atoms is expressed as energy levels of a particular value instead of energy bands or as energy bands in a very small area. Senior Researcher at Korea Electronics Technology Institute’s Display Materials & Components Research Center, Kyung-won Park, said, “In analogous terms, Quantum Dot electrons are comparable to a ball falling from the 10th floor to the 3rd, whereas electrons of luminescent materials used in other displays can be described as a ball falling from the range of 8th to 12th floor to the that of 1st to 5th floor.”
In the given depiction, if a ball fell from the 10th floor to the 3rd floor, that is exactly seven floors covered (a specific energy value), but in the latter, there are various possibilities (energy bands) - ranging from a ball that has fallen three floors to a maximum eleven floors. Tying it back to Quantum Dot technology, QDs can emit light closer to the intended wavelength compared to other light-emitting materials due to the existence of a range of atoms. "With the latest technology, Quantum Dot displays showcase the closest representation of true primary colors unlike any other conventional displays," according to Park. "This precise color expression capacity is the reason why Quantum Dot displays have the widest range of color in the display industry, producing a life-like image quality."

