
Phosphorescence is one of the key types of luminescence, including fluorescence, used in OLED displays. Luminescence is a phenomenon where light is given off when the energy level of the electrons in a light-emitting material drops from a high level (an excited state) to a low level (a ground state) as the reduced amount of energy is released in the form of light. Comprehension of the term fluorescence is necessary to understand phosphorescence.
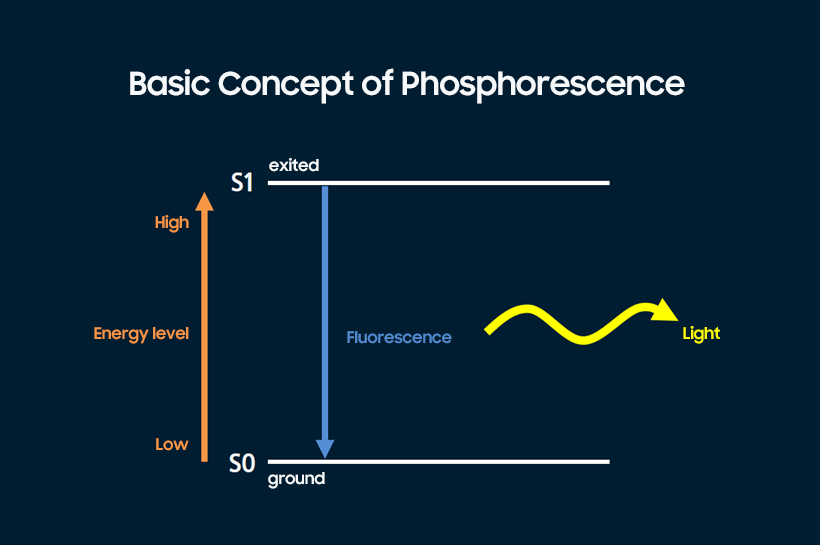
Fluorescence is a type of luminescence where energy is injected into a light-emitting material where the electrons are in a ground state so that the electrons become ‘excited,’ after which the energy level of the electrons drops to the more stable ‘ground’ state in a brief moment, releasing energy in the form of light, which is then used as the light source for a display. A potential major drawback with fluorescence is the limit of 25% singlet exciton production: The internal quantum efficiency of fluorescence is only 25% because of its inability to leverage triplet excitons that account for 75% of luminous energy. Phosphorescence has been developed as a more efficient type of luminescence.
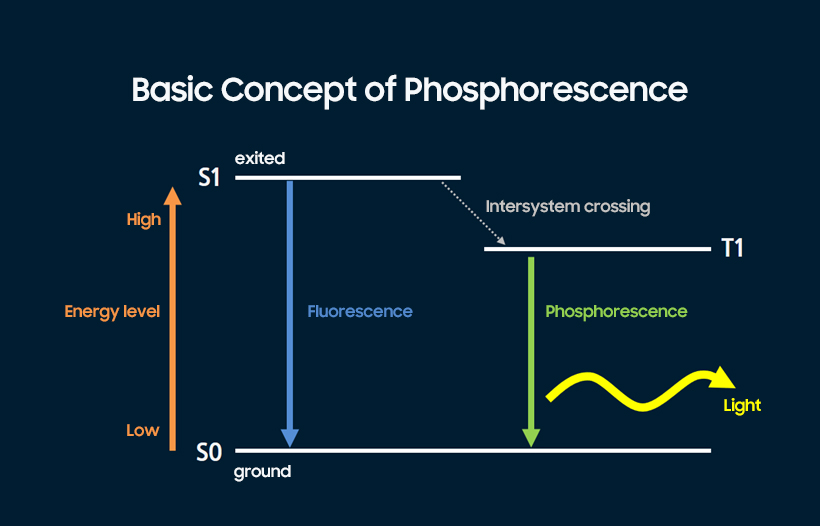
Phosphorescence, in essence, ensures that 75% of light emission from triplet-excited states wasted as heat or vibration in fluorescence is fully utilized. A light-emitting material that contains large, heavy metals such as complex compounds, including iridium (Ir) and platinum (Pt), tends to have a significantly larger orbital (three-dimensional space that electrons can inhabit) compared to regular organic matter. This means that the electrons are exposed to little impact from the pivot element, blurring the lines between triplet and singlet excitons. As such, the energy that can otherwise be used to emit light from an excited state indicated as S1 can be shifted to the T1 state in the illustration (intersystem crossing), enabling the triplet excitons to be used for light emission.
Phosphorescence theoretically offers the advantage of reaching 100% of internal luminous efficacy and was commercialized for green and red OLEDs. Blue, on the other hand, has the shortest wavelength among the RGB primary colors of light and requires the largest energy gap, which requires a higher level of stability. Therefore, ongoing research efforts are still to improve the lifespan of blue phosphorescence materials.



