
June 21 marks the tenth solar term in the annual cycle of 24 solar terms. Referred as the summer solstice in the northern hemisphere, it is the day with the longest daylight and when the sun reaches the highest altitude. On this day, daylight extends to a duration of 14 hours and 35 minutes. And due to the sun being at its highest point and the day being longer, it is a day with significant amount of exposure to the sunlight.
If you find yourself outdoors on this day, your primary concern should be UV rays. UV radiation consists of light with a wavelength shorter than 400 nm, carrying a significant amount of energy that can cause irritation to the human body. This is why it is crucial to apply sunscreen that offers protection against UV rays when venturing outside in the summer.

▲ Sunglasses with photochromic lenses (Source: Wikimedia Commons)
In addition to the skin, UV rays can also affect the eyes, leading to various conditions. They can cause “sunburn” on the cornea, similar to Ultraviolet Keratitis, trigger the onset of macular degeneration, and even raise the risk of cataracts. This is because the absorption of UV rays by the cornea raises the risk of inflammation. Surprisingly, despite these risks, a significant number of people do not wear sunglasses, especially those who already wear eye glasses. The inconvenience of carrying both eye glasses and sunglasses, as well as the need to constantly switch between them, contributes to this reluctance.
So why not have a pair of glasses that can serve as both sunglasses and eye glasses? Introducing Photochromic lenses, which are specifically designed to fulfill this dual purpose. These lenses darken when exposed to bright sunlight and regain transparency when sunlight is absent. Known as photosensitive lenses, photochromic lenses, or dimmable lenses, they possess the ability to change color in response to light.
Photochromic lenses, when were they invented?

▲ Eye protection goggles for nuclear test personnel (Source: Los Alamos National Laboratory, USA)
While photochromic lenses may seem like a cutting-edge technology because so few people use them, they have a long history. According to one theory, theses lenses were developed during the first half of the Cold War (1947-1968) in anticipation of potential use of nuclear weapons. The need for functional eyeglass lenses that could effectively shield against the intense light emitted from a nuclear explosion prompted research on protecting the eyes from the bright light of a nuclear blast.
Although the lenses were never used to protect the eyes from nuclear explosions, the research conducted during the Cold War period paved the way for the development of photochromic lenses. In 1964, the American glass manufacturer Corning Glass Works introduced the first glass photochromic lens. Subsequently, in 1970, the German lens manufacturer Zeiss Group and, in 1978, the British glass manufacturer Chance Pilkington entered the market with their own versions of photochromic lenses, leading to a competitive environment in the industry.
Despite the advancements, early technology had its limitations. Glass photochromic lenses are made from a mixture of silver halide (AgX), which is light-sensitive like silver chloride (AgCl). When exposed to UV light, these elements underwent a chemical reaction, causing the glass to darken. Although the change in the color was a win, the darkening process was slow. Furthermore, the use of glass material made the lenses expensive and heavy. Plus, the shape of the lenses had to be concave or convex, leading to variations in color depending on the thickness of each part. This posed a challenge in achieving the desired outcome of attaining a consistent color across the surface of the lens.

▲ Sunglasses by American Optical (Source: Wikimedia Commons)
This is how plastic photochromic lenses entered into the market. The first product, the Photolite photochromic lens, was introduced by American Optical in 1981. Although it was not successful, it sparked competition among lens manufacturers and spurred further research in plastic photochromic lenses. It wasn't until 1991 that photochromic lenses became commercially available, primarily due to the popularity of Transitions Optical products.
How do photochromic lenses pull off their impressive chameleon-like abilities?
At this point, you might be asking yourself: "What exactly causes these lenses to change color?"
Glass photochromic lenses utilize the light-sensitive properties of silver halide (AgX). When exposed to light, a photochemical reaction takes place in silver chloride (AgCl). This reaction is triggered by the energy from UV rays, which is sufficient to break chemical bonds. As a result, the silver ion obtains an electron from the chlorine ion, transforming into elemental silver (metallic silver), causing a color change to black (or brown if bromine or iodine is present instead of chlorine). Consequently, this reaction blocks UV rays and reduces the amount of light transmitted into the lenses.
Here is the equation (hv represents light)
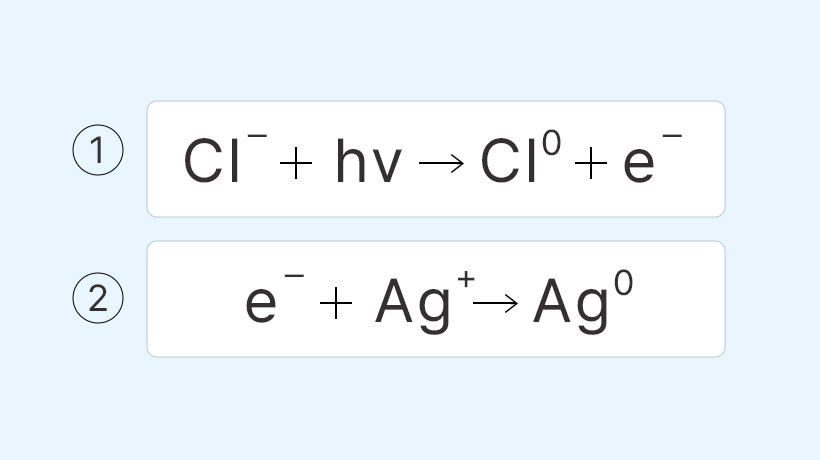
Silver chloride was previously utilized as a photosensitizer in photography and cinematic films. It is well known for its ability to darken upon exposure to light. However, in the case of photochromic lenses, the transformation required is not limited to blackening alone. The lenses must become transparent again. This seemingly difficult process involves a surprising simple solution: a reverse chemical reaction.
What helps is the copper ions (Cu+) added as a catalyst. When the UV light disappears, the lone chlorine ion steals an electron from the copper ion and turns it back into a chlorine ion. Since it has seven electrons, the chlorine atom's instinct is to grab another one to make a stable eight. The copper ion (Cu2+), which has lost an electron, takes the electron from the silver element and turns it into a silver ion, and the chlorine ion and the silver ion combine again to form silver chloride.
The key here is that since it's mixed in the glass, there are no additional ions apart from the copper ion to react with the silver and the remaining chlorine element. Therefore, the silver, having lost an electron to the copper, and the chlorine, having gained an electron from the copper, will react and revert back to the silver chloride state. It’s akin to a temporary separation of silver and chlorine ions caused by UV rays, wherein the copper intervenes and reunites them back together.
Plastic photochromic lenses employ different photochromic dyes, such as pyridobenzoxazines, naphthopyrans, and spiro-oxazine, instead of silver chloride. Upon exposure to UV light, these substances undergo molecular transformation that block UV light. Conversely, in the absence of UV light, they revert back to their initial molecular structure.
A must read for those looking to purchase sunglasses for summer vacation.
Early plastic photochromic lenses were produced by incorporating a photochromic substance directly into the plastic material. As a result, thicker lenses exhibited an imbalanced color appearance, similar to glass photochromic lenses. However, modern advancements have led to a different approach. Today, the photochromic material is applied as a coating on the surface of the plastic lens. The photochromic material is coated on top of the lens, followed by a protective and scratch-resistant coating.
Today’s photochromic lenses have significantly reduced time required for color transformation. Unlike their older counterparts that took about 10 minutes to darken and 30 minutes to regain their original color, today’s photochromic lenses tint in less than 30 seconds and fade in less than 6 minutes. This enhanced speed is because only the coating layer of the lenses needs to undergo the color change, rather than the glasses themselves. It is important to note that while these lenses are not capable of safeguarding your eyes in the event of a nuclear explosion, they are more than sufficient for everyday use.

▲ Application of photochromic plastic lenses to a motorcycle helmet visor (Source: Shoei)
Photochromic lenses are preferred by individuals seeking a convenient combination of eye eyeglasses and sunglasses or those with eyes that are sensitive to light. Cyclists, outdoor enthusiasts, and travelers commonly look for photochromic lenses. Additionally, there are photochromic lenses that respond to both visible and ultraviolet light, effectively blocking sunlight when UV rays are obstructed, such as inside of a car.
However, these lenses are not yet recommended for drivers because they create the effect of wearing sunglasses in a dark tunnel. They also have a lifespan of three to four years, and because they react to temperature and UV levels, they change colors more intensely in the winter than in the summer. Nevertheless, considering their convenience, it is worthwhile to consider photochromic lenses as an option when replacing your eye glasses in the future.

Author / Yoo Hoon Lee, IT Columnist
Former IAB Advisor Professor of the Department of Future & Tech at Hanyang University
Former expert member of the Tech Assessment Committee at the Korea Institute of S&T Evaluation and Planning
※ The views expressed in this article are personal opinions of the author and do not reflect the official position or strategy of Samsung Display Newsroom.

